Write the molecular orbital electronic distribution of oxygen specify its bond order and magnetic - Brainly.in

Write the molecular orbital electron distribution of oxygen `(O_(2))` Specify its bond order and - YouTube

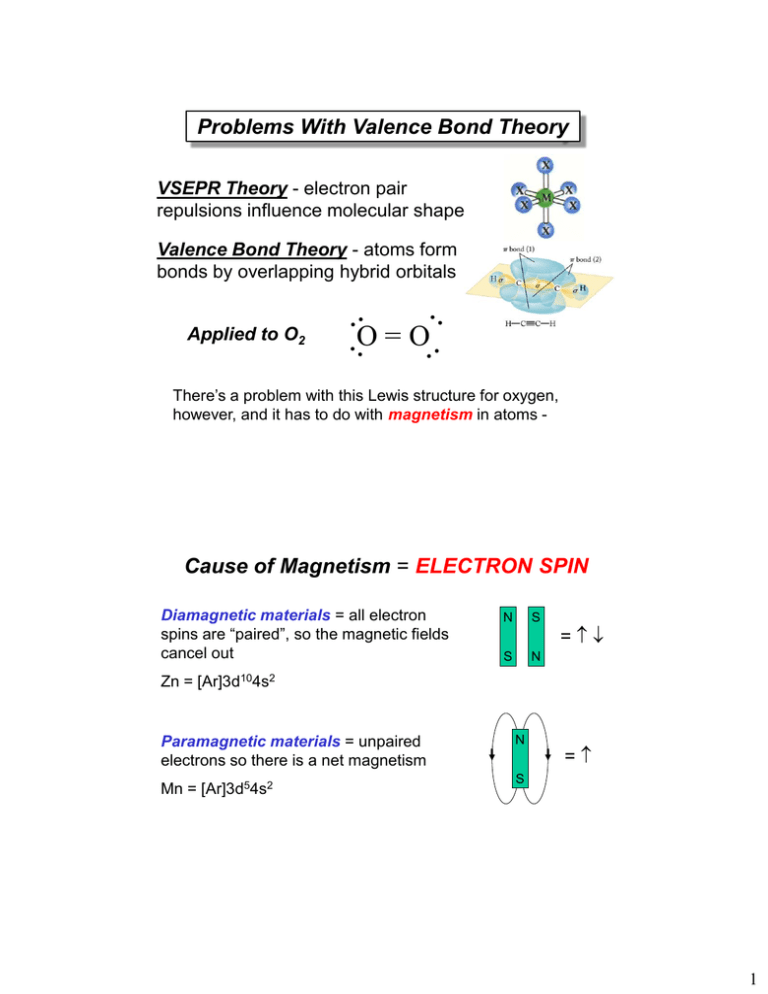
Molecular Orbital Theory Edward A. Mottel Department of Chemistry Rose-Hulman Institute of Technology. - ppt download

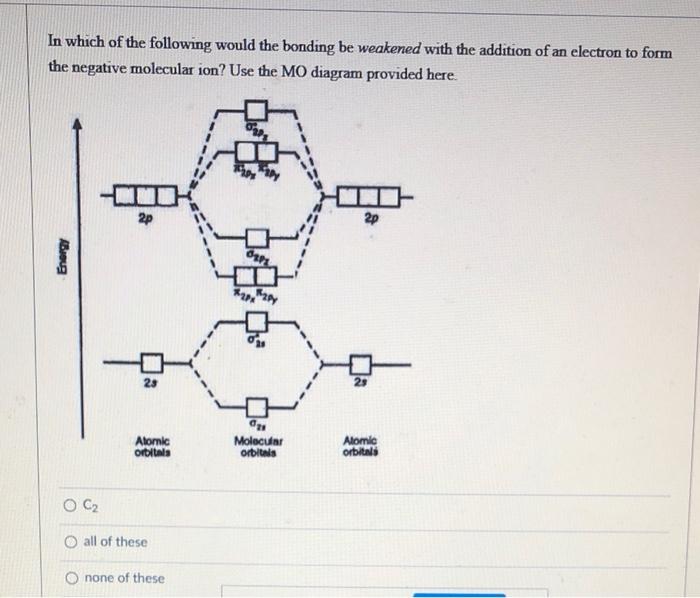
Compare the relative stability of the following species and indicate their magnetic properties : O2, O^+2, O2^ - (superoxide) and O2^2 - (peroxide).

Write magnetic properties of O2+, O2-, O22-,O2 - Chemistry - Chemical Bonding and Molecular Structure - 11117029 | Meritnation.com

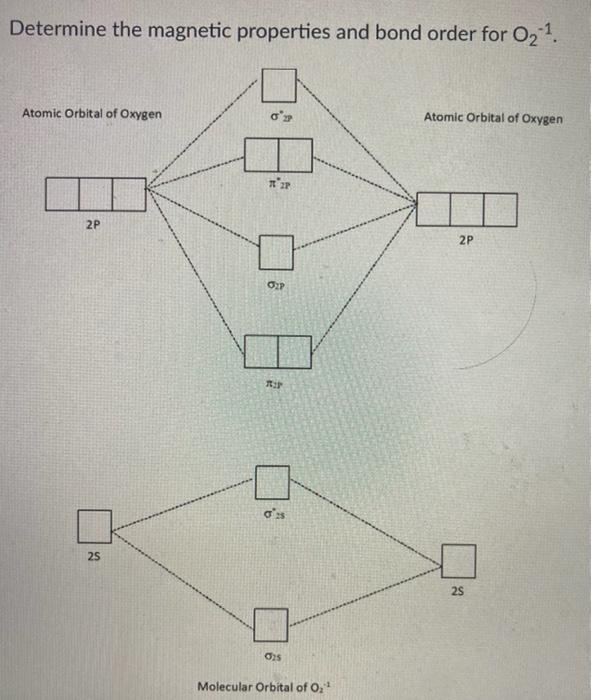
Based on molecular orbital theory compare the stability and magnetic property of o2+ and o2- - Brainly.in

Compare the relative stability of the following species and indicate their magnetic properties : O2, O^+2, O2^ - (superoxide) and O2^2 - (peroxide).

Compare the relative stability of the following species and indicate their magnetic properties: O(2),O(2)^(o+),O(2)^(ө)(superoxide),O(2)^(-2)(peroxoide).
![SOLVED:Considering the general Molecular Orbital diagram bel Ow; what would be the bond order and magnetic properties of CO? [Treat it in a similar fashion as N2, 02 etc__] Energy Bond order SOLVED:Considering the general Molecular Orbital diagram bel Ow; what would be the bond order and magnetic properties of CO? [Treat it in a similar fashion as N2, 02 etc__] Energy Bond order](https://cdn.numerade.com/ask_images/1d5da7974e074e1d990ccdde3fb578f1.jpg)
SOLVED:Considering the general Molecular Orbital diagram bel Ow; what would be the bond order and magnetic properties of CO? [Treat it in a similar fashion as N2, 02 etc__] Energy Bond order
Magnetic Properties of the Oxygen Molecule in Solid Oxygen‐Argon Mixtures: Journal of Applied Physics: Vol 40, No 3

Molecular Orbital Theory Edward A. Mottel Department of Chemistry Rose-Hulman Institute of Technology. - ppt download
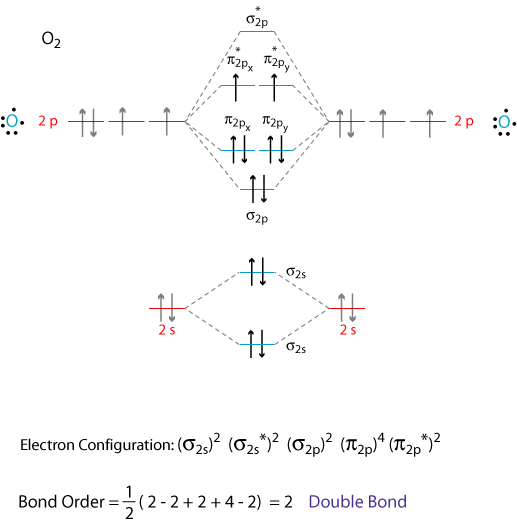
Write the Electronic configuration, Energy level diagram for the molecular orbitals of Oxygen molecule (O2). - Sarthaks eConnect | Largest Online Education Community